Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
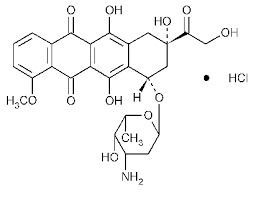
Sunitinib Malate Capsules
| Business Type | Exporter, Supplier |
| Form | Capsules |
| Treatment | Used to Treat Adults with Certain Types of Gastrointestinal Stromal Tumors, |
| Dosage | As per Doctor's Prescription |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
On November 16, 2017, the Food and Drug Administration approved sunitinib malate for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma following nephrectomy.On November 16, 2017, the Food and Drug Administration approved sunitinib malate (Sutent, Pfizer Inc.) for the adjuvant treatment of adult patients at high risk of recurrent renal cell carcinoma following nephrectomy.Sunitinib malate capsules belongs to a class of drugs called Antineoplastics, Tyrosine Kinase Inhibitor; Antineoplastics, VEGF Inhibitor.
A drug used to treat adults with certain types of gastrointestinal stromal tumors, pancreatic neuroendocrine tumors, or renal cell carcinoma (a type of kidney cancer). It is also being studied in the treatment of other types of cancer. Sunitinib malate blocks certain proteins, which may help keep cancer cells from growing. It may also prevent the growth of new blood vessels that tumors need to grow. Sunitinib malate is a type of tyrosine kinase inhibitor and a type of antiangiogenesis agent. Sunitinib is the active ingredient of sunitinib malate. Also called SU011248, SU11248, and Sutent. US Brand Name(s): Sutent. FDA Approved: Yes
Sunitinib, sold under the brand name Sutent, is a medication used to treat cancer. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) on January 26, 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.
Sunitinib malate is approved to treat adults with:
- Gastrointestinal stromal tumor (a type of stomach cancer). It is used in patients whose condition has become worse while taking imatinib mesylate or who are not able to take it.
- Pancreatic cancer. It is used in patients with progressive neuroendocrine tumors that cannot be removed by surgery, are locally advanced, or have metastasized (spread to other parts of the body).
- Renal cell carcinoma (a type of kidney cancer).
- Sunitinib malate is also being studied in the treatment of other types of cancer.
Looking for "Sunitinib Malate Capsules" ?
Explore More Products