Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
Atezolizumab Injection
| Business Type | Exporter, Supplier |
| API Form | Liquid |
| Usage | Treatment of Urothelial Carcinoma, Non-small Cell Lung Cancer |
| Dosage | As per Doctor's Prescription |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
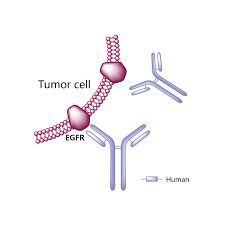
A drug that binds to the protein PD-L1 to help immune cells kill cancer cells better and is used to treat many different types of cancer, including cancers that express PD-L1. Atezolizumab is used alone or with other drugs to treat certain types of melanoma, hepatocellular carcinoma (a type of liver cancer), non-small cell lung cancer, small cell lung cancer, and urothelial cancer (a type of cancer in the bladder or urinary tract). It is also being studied in the treatment of other types of cancer. Atezolizumab may block PD-L1 and help the immune system kill cancer cells. It is a type of monoclonal antibody and a type of immune checkpoint inhibitor. Also called Tecentriq. US Brand Name(s): Tecentriq. FDA Approved: Yes
In the European Union atezolizumab is indicated for the treatment of urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma, and breast cancer.
In the United States, atezolizumab is indicated for the treatment of urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), hepatocellular carcinoma (HCC), and melanoma.
Atezolizumab is approved to treat:
- Hepatocellular carcinoma (a type of liver cancer) that is metastatic or cannot be removed by surgery. Atezolizumab is used with bevacizumab in patients who have not received systemic therapy.
- Melanoma that has a certain mutation in the BRAF gene. Atezolizumab is used with cobimetinib fumarate and vemurafenib in adults whose cancer is metastatic or cannot be removed by surgery.
- Non-small cell lung cancer.
- Small cell lung cancer. Atezolizumab is used with carboplatin and etoposide as the first treatment in adults with extensive-stage cancer.
- Urothelial cancer (a type of cancer in the bladder or urinary tract) that is metastatic or cannot be removed by surgery.
This use is approved under FDA’s Accelerated Approval Program. As a condition of approval, a confirmatory trial(s) must show that atezolizumab provides a clinical benefit in these patients.
Atezolizumab is also being studied in the treatment of other types of cancer.
Looking for "Atezolizumab Injection" ?
Explore More Products