Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
Lenvatinib Capsules
| Business Type | Exporter, Supplier |
| Dosage Form | Capsules |
| Treatment | Cancer |
| Dosage | As per Doctor's Prescription |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer.On April 7, 2021, the Food and Drug Administration granted regular approval to sacituzumab govitecan (Trodelvy, Immunomedics Inc.) for patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, at least one of them for metastatic disease.
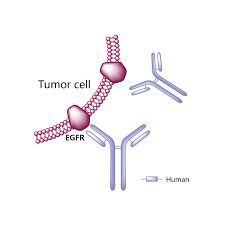
Sacituzumab govitecan (Trodelvy): In the case of this ADC, the monoclonal antibody part attaches to the Trop-2 protein on breast cancer cells and brings a chemo drug, similar to irinotecan, directly to them. (Some breast cancer cells have too much Trop-2, which helps them grow and spread quickly.)
This antibody-drug conjugate can be used by itself to treat advanced TNBC, after at least 2 other chemo regimens have been tried. This drug is given in a vein (IV) weekly for 2 weeks, followed by one week off, then restarted.
Looking for "Lenvatinib Capsules" ?
Explore More Products