Okhla, Delhi
- GST NO. : 07ABEFM6994Q2Z7
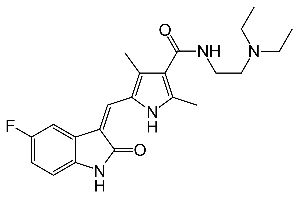
Eribulin Mesylate Injection
| Business Type | Exporter, Supplier |
| API Form | Liquid |
| Dosage | As per Doctor's Prescription |
| Type | Intravenous Administration |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
Storage
Cool and Dry Place
Best Before
36 Months from Manufacturing
On January 28, 2016, the U. S. Food and Drug Administration approved eribulin (HALAVEN injection, Eisai Co., Ltd.) for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
HALAVEN is a microtubule inhibitor indicated for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting .
Looking for "Eribulin Mesylate Injection" ?
Explore More Products